Exploring the Fundamentals of Reference Standards Characterization

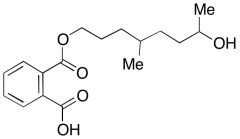
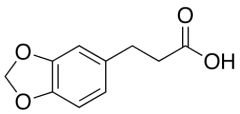
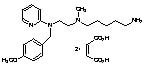
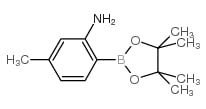
Reference standards in pharmaceuticals are meticulously defined materials or compounds that serve to determine the identity, potency, quality, and purity of various chemicals utilized in medical and pharmaceutical products. While pharmacopeias like the United States Pharmacopeia (USP), European Pharmacopoeia (EP), and Japanese Pharmacopoeia (JP) are primary sources, suppliers such as Clinivex provide precise reference standards to ensure the purity and quality of these chemicals.
The following assessments are typically employed to satisfy the essential tests for initial characterization:
Organic Impurities
In high-performance liquid chromatography (HPLC), ultraviolet (UV) detection is the most prevalent and extensively used detection method. Many organic impurities absorb in the UV spectrum, and HPLC can detect them, even though many solvents, especially those used in reversed-phase separations, are UV-transparent. The wavelength range can be extended to the visible light spectrum if necessary for detecting colored analytes.
Residual Solvents
Gas Chromatography — Flame Ionization Detection (GC-FID) is the most commonly utilized analytical technique in pharmaceutical research. The FID is highly sensitive to hydrocarbon impurities in the hydrogen and air supply for the flame, making GC-FID ideal for detecting residual solvents.
Water Content
Karl Fischer titration is a classic technique used to measure trace amounts of water in a sample using coulometric or volumetric titration. It offers several significant advantages over other moisture determination methods, including precision, speed, and specificity.
Metal Impurities
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) is a form of mass spectrometry capable of detecting metals on non-interfered low-background isotopes.
Non-volatile Impurities
The Residue on Ignition/Sulfated Ash test is a method for quantifying the amount of residual substance in a sample. This test is used to determine the quantity of inorganic contaminants present in an organic compound.
Furthermore, when creating reference standards, it is important to adhere to the following guidelines:
- High-purity materials and diluents are rigorously evaluated.
- Chromatographic purity is carefully assigned using various techniques.
- Residual impurities, such as water, inorganics, and solvents, are thoroughly analyzed.
- Validation methods ensure that solution concentration, purity, and stability are reliable and consistent.
- Minimum weighings are established to achieve a 0.1% relative inaccuracy on calibrated balances in their installed state.
- This uncertainty statement covers all aspects of standard preparation, from the characterization of neat material to the production of solutions.
In conclusion, reference standards play a crucial role in determining the purity of chemicals in pharmaceutical and medical products. It is essential that suppliers meet regulatory compliance before procurement. Therefore, selecting a top-notch reference standards supplier in Canada is vital to avoid any potential issues. For further details, visit Clinivex Reference Standards.
 info@theclinivex.com
info@theclinivex.com  +1 (877)-861-1996
+1 (877)-861-1996