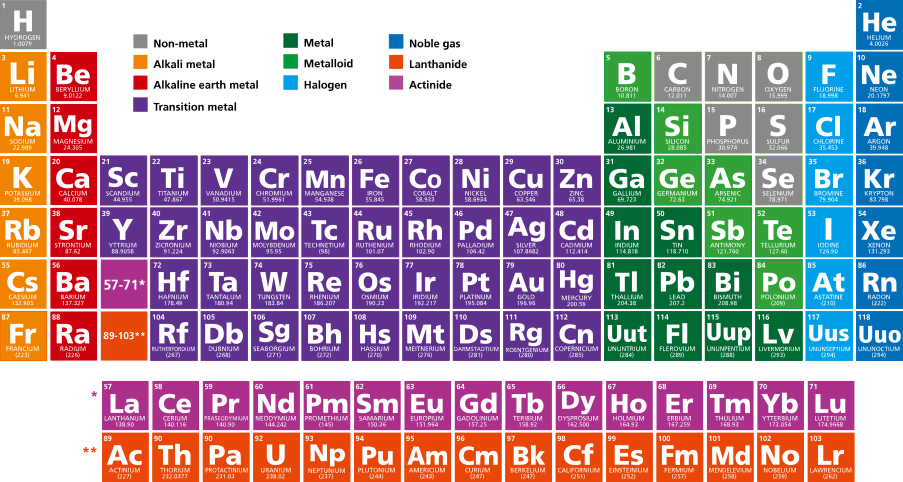
Periodic Table of Elements
The periodic table of elements is a systematic chart that organizes all known chemical elements based on their atomic number and electron configuration. It provides insights into the periodic trends and variations in elemental properties. The table includes essential details such as atomic number, atomic weight, electron configuration, and information on radioactivity, origin (whether natural or synthetic), as well as sometimes isotopic data, common oxidation states, and melting and boiling points.
The table is structured with horizontal rows called periods and vertical columns known as groups or families. Elements within the same period exhibit a gradual change in properties from metallic to non-metallic as you move from left to right. Elements in the same group share similar chemical characteristics. This arrangement helps scientists not only understand the relationships between known elements but also predict the properties of new or yet-to-be-discovered elements.
 Contact Us
Contact Us  info@theclinivex.com
info@theclinivex.com  +1 (877)-861-1996
+1 (877)-861-1996 


