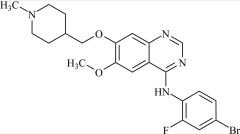
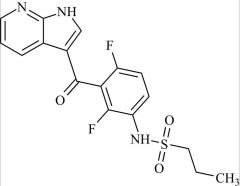
Pharmaceutical/API Drug Impurities/Metabolites
Clinivex provides a comprehensive selection of Pharmaceutical/API Impurities and Metabolites Reference Standards. These building blocks play a crucial role in drug development and chemical synthesis processes. Our reference standards are meticulously manufactured to meet the highest quality standards, ensuring reliability and accuracy in research and development activities. Trust Clinivex for premium-quality compounds that support the advancement of pharmaceutical science and innovation.
Categories
- Reference Standards
- Building Blocks
- Research Tools
- Environmental Standards
- Fluorescent Reagents
- Lipids/Fatty Acids
- Molecular Biology
- Miscellaneous Compounds
- Opiates
- Pesticides & Pesticide Metabolites
- Pharmaceutical
- Pharmaceutical/API Drug Impurities/Metabolites
- Phenethylamines/Amphetamines
- Spin Labels & Active Reagents
- Standards; Materials & Dyes
- Sulfhydryl Active Reagents
- Vitamin D Derivatives
- Standards
- Cannabidiol & Receptor
- Carbohydrates and Oligosaccharides
- Chiral Molecules
- Enzyme Activators and Inhibitors
- Fine Chemicals
- Glycerol Derivatives
- Isotopic Labeled
- Pharmaceutical/API Drug Impurities/Metabolites
- Phenethylamines/Amphetamines
- Nicotine Derivatives and Metabolites
- Nitrosamine
- Nitroimidazoles
- Semi-Synthetic
- Steroids
- Tranquilizer
- Natural Products
- Food and Beverage Standards
- Laboratory
- Glassware
- Plasticware
- Metalware
- Liquid Handling
- General Laboratory Products
- Life Science
- Chromatography
- Science Education
- Scientific Equipments
- Balances
- Bottle Top Dispenser
- Centrifuges
- Decapper
- Desiccators
- Hotplates & Stirrers
- Laboratory Timer
- Megnetic Stirrers
- Microscopes
- BioBlue Series Compound Microscopes
- bScope Series Compound Microscopes
- iScope Series Compound Microscopes
- Delphi-X Observer Compound Microscope
- Oxion Inverso Inverted Microscopes
- Delphi-X Inverso Inverted Microscope
- Trinocular stereo zoom microscope Stereo
- NexiusZoom Series Stereo Microscopes
- Microscope Accessories
- Pipettors
- Tube Rotators
- Tube Rockers
- Tube Rollers
- Vortex Mixer
- Serological Pipette Controller
- Primary Care
- Applied Science Products
All CategoriesClose
 info@theclinivex.com
info@theclinivex.com  +1 (877)-861-1996
+1 (877)-861-1996