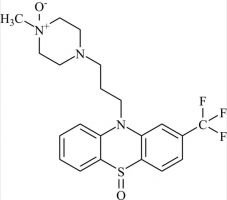
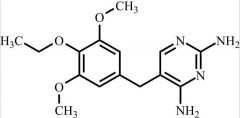
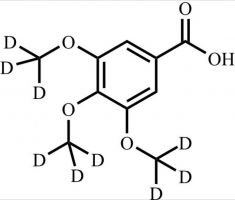
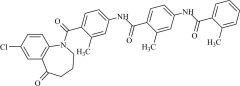
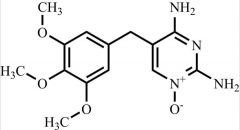
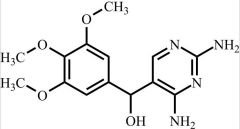
Pharmaceutical/API Drug Impurities/Metabolites
Clinivex provides a comprehensive selection of Pharmaceutical/API Impurities and Metabolites Reference Standards. These building blocks play a crucial role in drug development and chemical synthesis processes. Our reference standards are meticulously manufactured to meet the highest quality standards, ensuring reliability and accuracy in research and development activities. Trust Clinivex for premium-quality compounds that support the advancement of pharmaceutical science and innovation.
 info@theclinivex.com
info@theclinivex.com  +1 (877)-861-1996
+1 (877)-861-1996